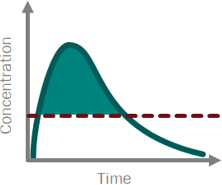
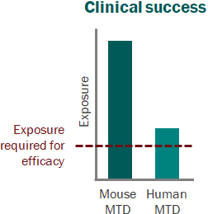
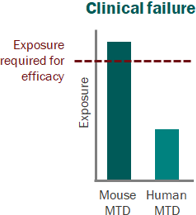
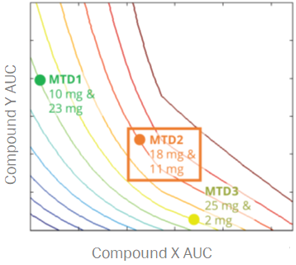
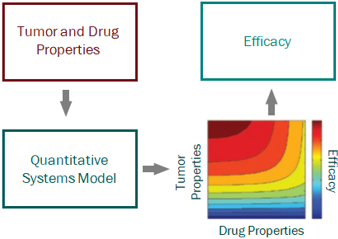
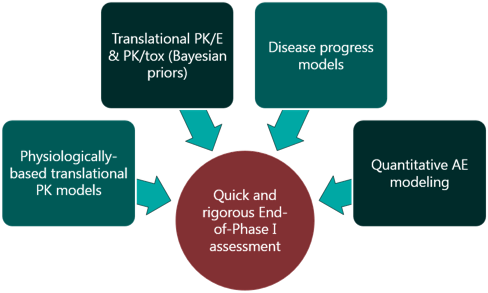
Skipping straight to the clinic: best practices for indications lacking validated preclinical efficacy models
Modeling tumor growth in animals and humans: an evolutionary approach (book chapter)
Directional inconsistency between response evaluation criteria in solid tumor (RECIST) time to progression and response speed and depth (published in Eur. J. Cancer)
Redeveloping to broaden the therapeutic window: the case study of Mylotarg
Population pharmacokinetics of immunity after COVID-19 infection and vaccination (preprint)
Using mixed-effects modeling to estimate decay kinetics of response to SARS-CoV-2 infection (published in Antibody Therapeutics)